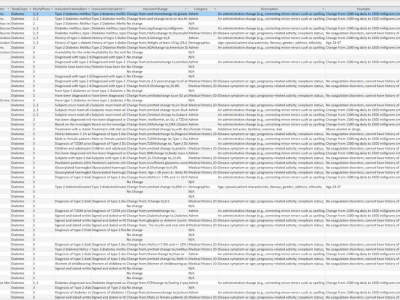
This dataset was created for an Eli Lilly and Company employee information management training program. It was part of a project that explored the potential use of ClinicalTrials.gov (CT.gov) as a tool to evaluate the severity of changes to inclusion and exclusion criteria on clinical trial operations. CT.gov is a public clinical study registry that records summary data about clinical trials. The registry includes a historical record of changes (change history) to inclusion and exclusion criteria and other data that is accessible by users.
- Categories:
