IR spectroscopy data of protein fibrils under THz irradiation
- Citation Author(s):
-
Antonia Intze (Sapienza University of Rome, Italy)Maria Eleonora Temperini (Sapienza University of Rome, Italy)Giorgio Gregori (Sapienza University of Rome, Italy)Valeria Giliberti (Istituto Italiano di Tecnologia, Rome, Italy)Federica Verde (Sapienza University of Rome, Italy)
- Submitted by:
- Michele Ortolani
- Last updated:
- DOI:
- 10.21227/h7cf-tg16
- Data Format:
 217 views
217 views
- Categories:
- Keywords:
Abstract
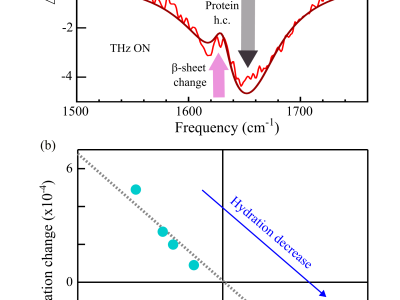
We investigated the effect of CW radiation at 0.6 THz on pathological protein aggregates in the form of amyloid fibrils, i.e. ordered protein complexes linked to neurodegenerative diseases such as Parkinson's and Frontotemporal Dementia. To monitor the effect of THz irradiation we exploited mid-infrared (mid-IR) vibrational spectroscopy in the amide-I band range, whose lineshape is known to depend on the protein conformation and on how proteins arrange into ordered supramolecular complexes such as fibrils. We coupled the focused THz beam to two different IR-based spectrometers: i) a conventional Fourier-transform IR (FTIR) Michelson interferometer where the estimated THz electric field is of the order of $\sim$ 1 $\frac{V}{cm}$; ii) an atomic force microscopy-assisted (AFM-IR) near-field spectrometer based on a tunable mid-IR quantum cascade laser, where much higher electric field ($\sim$ 0.1 $\frac{kV}{cm}$) is mainly achieved thanks to the field enhancement provided by the use of metallic AFM tip and sample support. In the first case, we interpreted the modification of the amide-I band upon THz irradiation in terms of an increase of the intermolecular forces within fibrils in response to environmental changes induced by THz irradiation (change of hydration). On the other hand, non-thermal effects are observed in the high-THz-field experiments performed on isolated fibril agglomerates in dry condition with the AFM-assisted spectrometer. The IR spectral response upon prolonged THz irradiation contains only the protein contribution and we obtain a different trend compared to the FTIR experiments, i.e. a weakening of the intermolecular forces, here directly induced by THz absorption and not mediated by changes of the environmental conditions. One can envision that further increase of the THz field value, such as with pulsed laser, can lead to the disassembly of protein fibrils.
Instructions:
infrared spectroscopy files, absorbance vs. wavenumbers (cm-1)